

Avoiding Flawed
Demonstrations:
Greenhouse Effect
Intro/Index
Faulty Demos
Errors, Misconceptions
Testing, Lab Results
Scientifically Strong
Resources
Rebuttals
Acknowledgements
Contact
Example of
Mobile Climate
Science Labs
Test Results:
Running
CLEAN'S
"Greenhouse Gas
in a Bottle
Demonstration"
Test after test:
there just
were not any
real, measurable
temperature differential
signals.

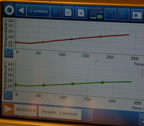

The air samples
were as
likely to
be slightly
higher than the CO2
as the other way around.



Various types of
incandescent lamps,
varying wattages



Calibration of
temperature probes.
All seven track together
within
0.2 Celsius from
5 to 60 degrees C.
Most within 0.05
C



Thank you,
Pasco Scientific
This web portal
is a resource within
America's online library
for Education &
Research
in Science,
Technology,
Engineering,
Mathematics:

|
 |
|
Three Independent Programs -- Lab Researchers & Science Educators
|

Climate change in a shoebox:
Right result, wrong physics
Published May 2010
in American Journal of Physics
American Association of Physics Teachers
Paper on the S.B. Lueddecke et al. demo:
Paul Wagoner -- TERC
Chunhua Liu -- Tufts University
and R. G. Tobin -- Tufts University
Available on-line on several servers:
Tufts.edu [pdf]
American Journal of Physics
Harvard.edu
Natural Science
Terc news announcement
Abstract of the Wagoner, Liu and Tobin paper
Classroom experiments that purport to demonstrate the
role of carbon dioxide’s far-infrared
absorption in
global climate change are more subtle than is
commonly appreciated. We show, using
both
experimental results and theoretical analysis,
that one such experiment demonstrates an entirely
different phenomenon: The greater density of
carbon dioxide compared to air reduces heat transfer
by suppressing convective mixing with the ambient air.
Other related experiments are subject to
similar concerns.
Argon, which has a density close to that of
carbon dioxide but no infrared
absorption,
provides a valuable experimental control for separating
radiative from convective
effects. A simple analytical
model for estimating the magnitude of the radiative
greenhouse effect is
presented, and the effect is
shown to be very small for most tabletop experiments.
Thank you, Scott Mandia,
for bringing this essential paper
to the attention of
the Mobile Climate Science Labs, immediately after we
contacted the Climate Science Rapid Response Team
for assistance on this topic.
|

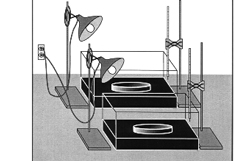
Mobile Climate Science Labs

Replicating the demos reviewed in this section.
Reviewing, reporting lab results.
Scientists, kids, teachers, engineers, parents.

Thermal imaging to test properties of
materials and energy sources commonly
used
in demos
at various IR wavelengths.

Bringing various demos out to the public.
Mainly it is the very solid, carefully evaluated demos.
Some always being vetted -- We want to let the kids,
parents and teachers have a chance to test them too.
Above: At the opening day of the Bill Nye Climate Lab
exhibit
at Chabot Space and Science Center.
Testing greenhouse in bottles & jars demos.
Sharing results, pro bono, with institutions
interested.

Back in the lab, doing quantative measurements
of the output of IR heat lamps --
as used by Bill Nye & Al Gore.
Illuminance/voltage dimming required, if to be equal.
|

Lawrence Hall of Science's:
Global Systems Science,
Lifelines for High School
Climate Change Education
A NASA Innovations in Climate Education project
Alan Gould, PI
   
Experimental results:
No differential temperature rise observed,
between concentrated CO2 and air.
Exposing samples side by side to sunlight.
Multiple runs, by independent groups,
using a variety of techniques
Quoting from:
From Teacher's Guide to the
GSS Climate Change Text
-- page 47
It occurred to several groups of teachers at the
Global Systems Science
institutes that an especially
valuable experience would be for students to measure
heat absorption by greenhouse gases.
The idea was certainly promising.
Scientists
at Mauna Loa Observatory used a heat
absorption method to accurately measure
the
concentration of carbon dioxide gas at a concentration of
just .035%. Surely,
a pure sample left in sunlight would
heat faster than a sample of air! This plan
was supported
by a published activity in which students measured heat
absorption
by water vapor, a greenhouse gas.
Unfortunately, all of the efforts by GSS staff and teacher
participants have
failed (so far) to develop a procedure,
using laboratory equipment that is easily available, that
will enable students to measure the differential absorption
of heat
energy by air and pure samples of greenhouse
gases. While the results seemed
reasonable in most of
the pilot experiments, the class data only turned up
random
differences in the temperatures of the various
samples. This was even the case
when we tested the
published activity.
We have speculated on several
reasons why it may be difficult to find
consistent
differences among the samples. Perhaps the gas samples
were too
small to absorb enough energy so that we could
measure a difference. Perhaps
heat was lost through the
walls of the containers. Perhaps our methods of
measurement were not sensitive enough. We won’t know
for sure, until we find
a method that works!
We still believe
that there’s a simple answer out there, somewhere, and
we
invite you to join in the search! To get you started, we’d
like to share the
excellent work done by the high school
teachers at the GSS institutes, so that
you can benefit from
their experience. If you develop a method that seems to
show consistent differences when the experiment is done
by an entire class of
students, please tell us how to do it!
|
|






















